Their changes in concentration are given by the column vector Δ a as. Chemistry chapter 8 9.

Reaction Yield An Overview Sciencedirect Topics
How to I calculate the entire yield from starting material to product.
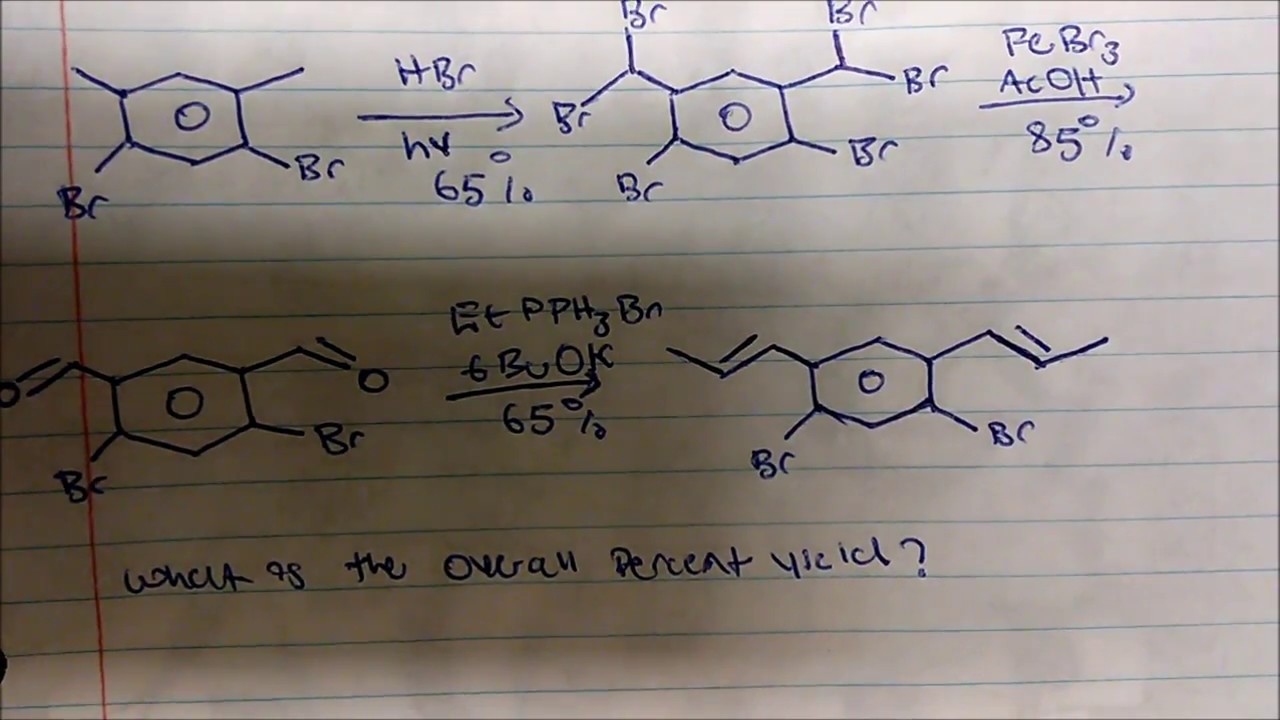
. Multiply the individual yields by each other. A simple explanation of a parallel reaction and how to calculate the yield and selectivity. In order to calculate the overall yield for a multistep process _____.
K NO 2 or k NO 2 O 2 The answer is k NO 2 since 2 molecules of NO and no O 2 are involved in the reaction prior to the slow step. So for example if a synthesis has two steps each of yield 50 then the overall yield is 50 x 50 25. N 2 O 2 g O 2 2NO 2 g fast Which is the most likely rate law for the reaction.
Overall yield of a multistep reaction percent. I2 s Cl2 g--2ICl s and. Reactant rarr Product moles of productmoles of reactant xx 100 Yield Of course you need a balanced chemical equation and specific quantities of reactant.
0875 x 0912 x 0793 x 0819 x 100 518 overall. DO NOT FORGET TO SUBSCRIBEThis video puts emphasis on calculating the overall percent yield of an Organic Synthesis. 819 The overall percent yield is computed as shown here.
If this is theoretical then yes overall the reaction is 11 the whole way through so the number of moles of bromobenzene is how many moles of benzoic acid you should have. To link to this Multistep Reactions page copy the. Divide the actual yield made in the lab by the calculated theoretical amount and multiply by 100.
Post lab questions show the detailed mechanism for the reaction calculate the yield. Often one reactant is present in excess and the other reactant is the limiting reagent. Multi-Step Synthesis of Methyl m-Nitrobenzoate AEM last update July 2013 This Fisher Esterification is the final step of the multi-step synthesis of methyl m-nitrobenzoate.
A simple explanation of a parallel reaction and how to calculate the yield and selectivity. 25 x ν 1 Δ a. Yield 100 x moles of product obtained maximum number of moles of product possible Note that if a synthesis is a linear multistep process then the overall yield is the product of the yields of each step.
The first step showed a percent yield of 64 and the second step a yield of 56. Reaction Yield An Overview Sciencedirect Topics Pin By Threefourthsme On Chemistry Covalent Bonding Teaching Style Classwork Solved Multi Step Synthesis Yield Calculation When You Chegg Com. To determine the percent yield.
For a synthesis to find the overall percent yield multiply the individual percent yields of every step by each other ex. Calculate the yield of each step and then multiply them together. Multiply the value you get by 100 in order to get the value of the actual yield.
Make sure to use the same unit of measurement for both values. Whats the purpose of adding sodium sulfate. How do you calculate the total yield of a multistep reaction.
In order to calculate the overall yield for a multistep process _____-multiply the individual yields by each other. And calculate your overall yield based on your experiment and this experiment. 3 steps all 30 yield 030 x 030 x 030 027 x 100 27 overall.
Sets with similar terms. Yield from A to B Yield from B to C. Endgroup Gaurang Tandon Jan 4.
Calculate the percentage yield for the reaction. Chemists would gladly accept yields of 80-90. In organic chemistry many complex reactions occur that are multi-step also.
Alternatively you can take the limiting reagent molar amount of. This metric attempts to define yield in terms of that proportion. Iodine trichloride is produced in a two step process represented by the following balanced equations.
In this multistep synthesis the starting reagent is aniline propose how to make 4-Bromacetanilde from aniline. Effective mass yield Hudlicky et al6 proposed a metric known as effective mass yield that is defined as the percentage of the mass of desired product relative to the mass of all non-benign materials used in its synthesis Or stated mathematically. Yield Actual YieldTheoretical Yield100 If you now want to calculate the overall yield you have to multiply all the parcial yields.
If needed convert one of the values before performing the calculation. Abbreviated versions of the three reaction steps are given here with more complete versions of the overall reactions given with each lab prompt separately. The overall yield of a multi-step reaction composed of various single steps is calculated by multiplying the partial yields for each of the single-step reactions converting all the percentages to fractions of 100 or to decimals and multiply them.
Express this value as a percentage of the. Calculate your overall percent yield for the multi-step reaction. To calculate the overall percentage yield of a multi-step organic reaction multiply the individual percentage yields of every step by each other for example for 3 steps 30 yield in every step we can calculate 030 times 030 times 030 027 times 100 27 overall.
In the case of a multi-step mechanism the matrix v thus includes only the stoichiometric coefficients of the reactants selected and taking part in all the different steps of the reaction. How To Calculate Overall Yield Of Multi Step Synthesis Total Synthesis. Yes the rule is that the final rate law expression for a multi-step reaction should not contain any term for the intermediates in it.

How To Calculate Overall Yield Of Multi Step Synthesis Total Synthesis

Calculating The Overall Percent Yield Of A Synthesis Organic Chemistry Youtube

0 Comments